 Early Detection of Cerebral Palsy
Early Detection of Cerebral Palsy
- Section I: Evidence Summary
- Section II: Selected Published Evidence
- Section III: Practical Tools
- Section IV: Acknowledgments
- Feedback/
Comments
Section I: Evidence Summary Printer Friendly Version
A. Definitions
Cerebral palsy has traditionally been diagnosed between 12-24 months of age because there is no laboratory biomarker for cerebral palsy. Cerebral palsy is a clinical diagnosis, diagnosed based on a combination of clinical signs, neurological symptoms and physical limitations. Late diagnosis means some infants do not receive early intervention when they would benefit most. Cerebral palsy or high-risk of cerebral palsy can now be detected accurately and early using a combination of standardized assessment tools. Early detection enables timely early intervention when the greatest gains are possible from neuroplasticity.
B. Why is Early Detection Important?
Cerebral palsy should be detected as soon as possible because:
- Cerebral palsy specific early intervention using intense, motor learning task-specific approaches plus environmental enrichment optimizes natural plasticity and improves children’s motor and cognitive outcomes.
- Early, regular monitoring and treatment for the known musculoskeletal complications of cerebral palsy can prevent the onset of hip dislocation, scoliosis and contracture.
- Parents experience more depression and stress when they are dissatisfied with the diagnostic process. Families prefer early diagnosis, followed by early intervention and parent-to-parent support.
It is not good practice to offer conservative “wait and see” monitoring, when clear clinical diagnostic indicators exist, especially in contexts where the absence of a diagnostic label precludes the infant from accessing the recommended early intervention. There is evidence that delayed diagnosis worsens parental mental health (Baird et al, 2000) and clinical trial evidence is emerging that the lack of intense early intervention may restrict the infant’s motor and cognitive gains (Morgan et al, 2016). Neuroscience evidence indicates that brain development and refinement of the motor system continues in the postnatal period, driven by activity in the motor cortex (Eyre et al, 2014; Martin et al, 2011). Early active movement and intervention is essential because infants not actively using their motor cortex risk losing cortical connections and dedicated function (Eyre et al, 2014; Martin et al, 2011). Furthermore, there is increasing evidence that the infant’s motor behavior, through discovery and interaction with the environment, controls and generates the growth and development of muscle, ligament, and bone, as well as driving the ongoing development of the neuromotor system. These recent discoveries about brain and muscle plasticity support the earliest possible intervention to: (a) exercise muscles through their functional length (as muscles grow throughout development in response to the infant’s actions); and (b) train specific actions, in order to promote motor learning and ‘drive’ plasticity and effective functional motor performance (Eyre et al, 2014; Martin et al, 1999; Shepherd 2014).
Randomized controlled trial (RCT) data is beginning to indicate that infants with unilateral/hemiplegic cerebral palsy, who receive early Constraint Induced Movement Therapy (CIMT) have better hand function than controls short-term and probably substantially better hand function long-term (Eliasson et al, 2015). Population register data indicates that children with bilateral cerebral palsy, who receive regular surveillance and intervention have lower rates of hip displacement, contracture and scoliosis complications (Elkamil et al, 2011; Hägglund et al, 2005; Scrutton et al, 2001). Hip Surveillance guidelines can be found on the care pathways website. RCT data is also beginning to indicate that infants with any type and topography of cerebral palsy, who receive “GAME” (Goals – Activity – Motor Enrichment, which is an early, intense, enriched, task-specific, training-based interventions at home), have better motor and cognitive skills at 1-year, than those who received usual care (Morgan et al, 2016). Importantly, RCTs also suggest that, improvements are even better when training occurs at home (Novak et al, 2009; Rostami et al, 2012) because children learn best in supported natural settings, where training is personalized to their enjoyment – translating to more intense, specific and relevant practice. Task-specific, motor learning training-based early intervention (e.g. GAME and CIMT) are recommended as the new paradigm of care for infants with cerebral palsy as they induce neuroplasticity and produce functional gains (Morgan et al, 2016b). Larger replication studies are underway, meaning more evidence will inform our estimate in the confidence of the effects.
C. Target Population
Infants with cerebral palsy and their parents.
D. Target Clinical Providers
Neurologists, pediatricians, neonatologists, pediatric rehabilitation specialists/physiatrists, general practitioners, neuro-radiologists, physiotherapists, occupational therapists, speech pathologists, nurses and early educators.
E. Early Detection Strategies
Evidence indicates that there are two major pathways to accurate and early detection of cerebral palsy depending on the infant’s age at the time of assessment using different tests in combination with the clinical examination.
- For infants younger than 5-months of age (corrected for prematurity) known as the “Newborn Detected Risks” pathway: abnormal motor function detected as “absent fidgety” on Prechtl’s General Movements Assessment (GMs) plus an abnormal brain Magnetic Resonance Imaging (MRI) indicating damage to the motor area/s accurately predicts cerebral palsy more than 95% of the time and is strongly recommended. The Test of Infant Motor Performance (TIMP) can also be used as it predicts cerebral palsy 61-90% of the time. NOTE: Each test has excellent sensitivity and specificity in isolation, but the pooled predictive power of three tests is even higher for an early accurate diagnosis of cerebral palsy. The combined predictive power of neuroimaging plus HINE plus absent fidgety GMs is a sensitivity and specificity value of 97.86% and 99.22% (PPV 98.56%, NPV 98.84%) (Morgan et al, 2019). Key Neuroimaging Evidence: PRE-TERM infants: Term equivalent age (TEA) (or as close as possible) MRI is most predictive of outcome (Reid et al, 2014). Where possible, use a 3 Tesla (3T) scanner to improve the ability to detect subtle lesions. Sequential cranial ultrasound (CUS) can also predict non-ambulatory cerebral palsy but may fail to detect subtle lesions commonly associated with diplegia. When an MRI is performed within a week after a presumed insult, diffusion weighted imaging (DWI) can be predictive of subsequent cystic evolution in the white matter (de Vries et al, 2015; Kwon et al, 2014; Woodward et al, 2006). TERM-BORN infants: MRI in the first week of life is recommended for infants born at term with suspected brain abnormalities. If the infant has had encephalopathy, conventional MR sequences may not show any signs of abnormality in the first 48-hours Diffusion weighted imaging (DWI) and apparent diffusion coefficient maps (ADC) are likely to detect the injury early. Waiting 3-5 days before imaging is recommended, to maximize identifying abnormal findings. Conventional T1 beyond the first week and DWI before the end of the first week may also allow examination of the posterior limb of the internal capsule (PLIC) and the descending corticospinal tracts at the level of the cerebral peduncles, which is highly predictive of permanent motor dysfunction (Cowan et al, 2005; Kirton et al, 2007). If myelination asymmetry can be visualized this is highly predictive of hemiplegic cerebral palsy (de Vries et al 2001).
- For infants older than 5-months of age (corrected for prematurity) known as the “Infant Detected Risks” pathway: A Hammersmith Infant Neurological Evaluation (HINE) score lower than 73 (at 6, 9 or 12 months) (scored neurological clinical examination) plus an abnormal brain MRI indicating damage to the motor area/s accurately predicts cerebral palsy 90% of the time and is strongly recommended. The Developmental Assessment of Youth and Children (DAYC) (parent-report scored checklist) can also be used as it accurately predicts cerebral palsy 80% of the time. The Alberta Infant Motor Scale (AIMS) and the Neuro Sensory Motor Development Assessment (NSMDA) can be used as supplementary assessments for predicting an abnormal motor outcome, 86% and 82% predictive respectively. Parent concern is a valid reason to trigger formal diagnostic investigations and referral to early intervention, as eighty-six per cent of parents suspect their child has cerebral palsy before the clinical diagnosis is made (Baird et al, 2000). NOTE: High-quality early detection studies of lower-risk term-born infants without early discernible indicators of cerebral palsy do not exist in the literature, as these infants are more difficult to identify and study very early. Therefore, the recommendations for early detection of cerebral palsy in infants older than 5-months are conditional (or weaker strength) recommendations based on best-available evidence, which is extrapolated from high-risk populations. We chose to make this extrapolation because despite the differing causal pathways to cerebral palsy, population data indicates that lower-risk cases have comparable type and topography cerebral palsy profiles to high-risk cases, but with more severe motor impairments. Key DAYC evidence: A large retrospective study of n=606 high-risk neonatal intensive care graduates (preterm <1500g or neonatal encephalopathy), found that a drop of 2 standard deviations in DAYC motor scores between 6-12 months is 89% predictive of cerebral palsy (Maitre et al, 2013).
In Low to Middle Income Contexts (LIMC) or where MRI is not safe, feasible or affordable, the most reliable alternatives are: the HINE (scored neurological clinical examination) and the DAYC (parent-report scored checklist) which accurately predicts cerebral palsy 90% and 80% of the time respectively. NOTE: In contexts where an MRI is not available, safe, feasible or affordable, then detection of cerebral palsy with “infant detectable risks” and older than 5-months of age but less than 24-months of age is still possible using standardized tools and should be carried out to enable access to early intervention. On the algorithm this care pathway, is referred to as option B. Early detection of risk of cerebral palsy in 5-24 months olds without an MRI is most accurately conducted using the: (a) Hammersmith Infant Neurological Evaluation (HINE) detects abnormal neurological dysfunction and is 90% predictive of cerebral palsy from 2-24 months of age. HINE scores at 6, 9 or 12 months, where: <73 indicates risk of cerebral palsy; and <40 indicates abnormal outcome, usually cerebral palsy; (b) Developmental Assessment of Young Children (DAYC) to quantify motor delay (89% predictive of cerebral palsy); and (c) Movement Assessment of Infants (MAI) to quantify motor delay (73% predictive of cerebral palsy in high-risk infants at 8 months of corrected age). Key evidence: A large prospective cohort study of high-risk infants (preterm or term with encephalopathy) were studied from birth until 12 months corrected age and the global HINE scores were found to predict cerebral palsy with >90% accuracy (Pizzardi et al, 2008). Systematic review evidence of tools to detect cerebral palsy, found moderate quality evidence that the MAI can detect cerebral palsy with 96% accuracy at 8-months post term age, in infants with high-risk for cerebral palsy (Spittle et al, 2008).
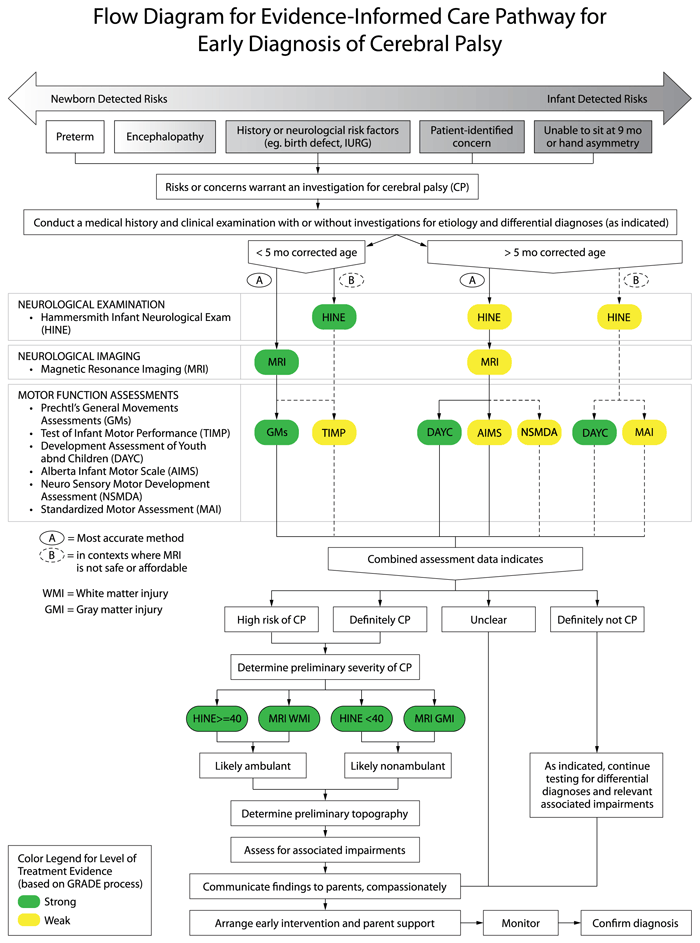
Reproduced with permission from JAMA Pediatrics. 2017. 171(9): Figure 1. Copyright© 2020 American Medical Association. All rights reserved.
Section II: Selected Published Evidence
Novak I, Morgan C, Adde L, Badawi N, Blackman J, Boyd R, Cioni G, Damiano D, Darrah J, deVries L, Einspieler C, Fehlings D, Ferrerio D, Fetters L, Forssberg H, Gordon A, Guzzetta A, Karlsson P, Maitre N, McIntyre S, Noritz G, Pennington L, Romeo D, Shepherd R, Valentine J, Walker K, White R (2017). Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatrics, 171(9), 897-907.
Recommended Reading:
Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, et al. Practice Parameter: Diagnostic assessment of the child with cerebral palsy. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurol 2004; 62: 851-63.
Bosanquet M, Copeland L, Ware R, Boyd R. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 2013; 55: 418-26.
Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: Neuroimaging of the neonate Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurol 2002; 58: 1726-38.
Romeo DM, Ricci D, Brogna C, Mecuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol 2015, 58(3), 240-245.
References:
Baird G, McConachie H, Scrutton D. Parents' perceptions of disclosure of the diagnosis of cerebral palsy. Arch Dis Child 2000; 83: 475-80.
Cowan FM, de Vries LS. The internal capsule in neonatal imaging. In Semin Fetal Neonatal Med 2005; 10: 461-474. WB Saunders.
de Vries LS, Roelants-van Rijn AM, Rademaker KJ, van Haastert IC, Beek FJ, Groenendaal F. Unilateral parenchymal haemorrhagic infarction in the preterm infant. Eur J Paediatr Neurol 2001; 5: 139-49.
de Vries LS, Benders MJ, Groenendaal F. Progress in Neonatal Neurology with a Focus on Neuroimaging in the Preterm Infant. Neuropediatrics 2015; 46: 234-41.
Eliasson AC, Holmefur M. The influence of early modified constraintâ€induced movement therapy training on the longitudinal development of hand function in children with unilateral cerebral palsy. Dev Med Child Neurol 2015; 57: 89-94.
Elkamil AI, Andersen GL, Hägglund G, Lamvik T, Skranes J, Vik T. Prevalence of hip dislocation among children with cerebral palsy in regions with and without a surveillance programme: a cross sectional study in Sweden and Norway. BMC Musculoskelet Disord 2011; 12: 284.
Eyre J. Corticospinal tract development and activity dependent plasticity. In R Shepherd (Ed). Cerebral palsy in infancy. Oxford: Elsevier; 2014. 53-66.
Hägglund G, Andersson S, Düppe H, Lauge-Pedersen H, Nordmark E, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy the first ten years of a population-based prevention programme. J Bone Joint Surg Br 2005; 87: 95-101.
Kirton, A., Shroff, M., & Visvanathan, T. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke 2007;38:974-980.
Kwon SH, Vasung L, Ment LR, Huppi PS. The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clin Perinatol 2014; 41: 257-83.
Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev 2013; 89: 781-6.
Martin JH, Chakrabarty S, Friel KM. Harnessing activity-dependent plasticity to repair the damaged corticospinal tract in an animal model of cerebral palsy. Developmental Medicine & Child Neurology. 2011;53:9-13.
Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res 1999; 125: 184-99.
Morgan CJ, Novak I, Dale RC, Guzzetta A, Badawi N. Single blind randomised controlled trial of GAME (Goals - Activity - Motor Enrichment) in infants at high risk of cerebral palsy. Res Dev Disabil 2016; 55:256-267.
Morgan C, Darrah J, Gordon AM, Harbourne R, Spittle A, Johnson R, Fetters L. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol 2016b; 58(9), 900-909.
Morgan C., Romeo D., Chorna O., Novak I., Galea C., Del Secco S. & Guzzetta A. (2019). The pooled diagnostic accuracy of three tests for diagnosing cerebral palsy early in high risk infants: a case control study. Journal of Clinical Medicine (In Press).
Novak I, Cusick A, Lannin N. Occupational therapy home programs for cerebral palsy: double-blind, randomized, controlled trial. Pediatr 2009; 124(4), e606-e614.
Reid SM, Dagia CD, Ditchfield MR, Carlin JB, Reddihough DS. Populationâ€based studies of brain imaging patterns in cerebral palsy. Dev Med Child Neurol 2014; 56: 222-32.
Rostami HR, Malamiri RA. Effect of treatment environment on modified constraint-induced movement therapy results in children with spastic hemiplegic cerebral palsy: a randomized controlled trial. Disabil Rehabil 2012; 34(1), 40-44.
Pizzardi A, Romeo DM, Cioni M, Romeo MG, Guzzetta A. Infant neurological examination from 3 to 12 months: predictive value of the single items. Neuropediatrics 2008; 39: 344-6.
Scrutton D, Baird G, Smeeton N. Hip dysplasia in bilateral cerebral palsy: incidence and natural history in children aged 18 months to 5 years. Dev Med Child Neurol 2001; 43: 586-600.
Shepherd RB. (Ed). Cerebral palsy in infancy: Targeted activity to optimize early growth and development. Oxford: Elsevier Health Sciences, 2014.
Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol 2008; 50: 254-66.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006; 355: 685-94.
Section III: Practical Tools
Plain Language Summary

Parent Fact Sheets and an Infographic Video are available here:
https://www.cerebralpalsy.org.au/about-conditions/cerebral-palsy/early-diagnosis/
Training in the General Movement’s Assessment dates and locations can be found here:
http://general-movements-trust.info/47/dates
A Continuing Medical Education e-learning program is being built for professionals to provide in-depth information on how to diagnose early. To learn more or express an interest in obtaining more information contact: Lynda McNamara Lynda.McNamara@health.qld.gov.au.
Section IV: Acknowledgments
Stakeholder Consultation:
Three families with children aged between 3 months and 6 years-of-age with varying degrees of hypotonia reviewed the pathway and provided written feedback that was incorporated into the care pathway.
Expert Consensus Team:
| Name | Affiliation(s) | Location | Specialty Expertise |
|---|---|---|---|
| Iona Novak | Cerebral Palsy Alliance, The University of Sydney | Sydney, NSW, Australia | Occupational Therapist |
| Cathy Morgan | Cerebral Palsy Alliance, The University of Sydney | Sydney, NSW, Australia | Physical Therapist |
| Lars Adde | Norwegian University of Science and Technology; St Olavs University Hospital | Trondheim, Norway | Physical Therapist |
| Nadia Badawi | Cerebral Palsy Alliance, The University of Sydney, Children’s Hospital Westmead The University of Sydney | Sydney, NSW Australia | Neonatologist |
| James Blackman | Cerebral Palsy Alliance Research Foundation | New York, NY, USA | Pediatrician |
| Roslyn N Boyd | The University of Queensland | Brisbane, QLD, Australia P | hysical Therapist |
| Janice Brunstrom-Hernandez | Children’s Medical Centre, Dallas | Dallas, TX, USA | Child Neurologist |
| Giovanni Cioni | University of Pisa, Stella Maris Scientific Institute | Pisa, Italy | Child Neurologist & Psychiatrist |
| Diane Damiano | National Institutes of Health | Washington, DC, USA P | hysical Therapist |
| Johanna Darrah | Faculty of Rehabilitation Medicine, University of Alberta | Alberta, Canada | Physical Therapist |
| Ann-Christin Eliasson | Karolinska Institutet | Stockholm, Sweden | Occupational Therapist |
| Prof Linda de Vries | University Medical Centre, Utrecht | Utrecht, The Netherlands | Neonatologist & Neurologist |
| Christa Einspieler | Medical University of Graz | Graz, Austria | Physiologist |
| Michael Fahey | Monash University, Australia | Melbourne, Vic, Australia | Neurologist & Geneticist |
| Darcy Fehlings | Holland Bloorview Kids Rehabilitation Hospital, University of Toronto | Toronto, ON, Canada | Developmental Pediatrician |
| Donna Ferriero | University California San Francisco | San Francisco, CA, USA | Child Neurologist |
| Linda Fetters | University of Southern California | Los Angeles, CA, USA | Physical Therapist |
| Simona Fiori | Stella Maris Scientific Institute, Pisa | Pisa, Italy | Child Neurologist & Psychiatrist |
| Hans Forssberg | Karolinska Institutet | Stockholm, Sweden | Neuroscientist |
| Andrew Gordon | Teachers College Columbia University | New York, NY, USA | Neuroscientist |
| Susan Greaves | The Royal Children’s Hospital, Melbourne, Australia | Melbourne, Vic, Australia | Occupational Therapist |
| Andrea Guzzetta | University of Pisa, Stella Maris Scientific Institute, Pisa | Pisa, Italy | Child Neurologist & Psychiatrist |
| Mijna Hadders-Algra | University of Groningen, University Medical Center Groningen, Dept Pediatrics | Groningen, The Netherlands | Developmental Neurologist |
| Regina Harbourne | Duquesne University | Pittsburgh, PA, USA | Physical Therapist |
| Angelina Kakooza-Mwesige | Makerere University | Kampala, Uganda | Neurologist |
| Petra Karlsson | Cerebral Palsy Alliance, The University of Sydney | Sydney, NSW, Australia | Occupational Therapist |
| Lena Krumlinde-Sundholm | Karolinska Institutet | Stockholm, Sweden | Occupational Therapist |
| Beatrice Latal | University Children’s Hospital Zurich | Zurich, Switzerland | Pediatrician |
| Alison Loughran-Fowlds | Children’s Hospital Westmead, The University of Sydney | Sydney, NSW, Australia | Neonatologist |
| Nathalie Maitre | Nationwide Children’s Hospital, The Ohio State University | Columbus, OH, USA | Neonatologist |
| Sarah McIntyre | Cerebral Palsy Alliance, The University of Sydney | Sydney, NSW, Australia | Epidemiologist |
| Garey Noritz | Nationwide Children’s Hospital, The Ohio State University, USA | Columbus, OH, USA | Pediatrician |
| Lindsay Pennington | Newcastle University | Newcastle, UK | Speech Language Pathologist |
| Domenico M. Romeo | Pediatric Neurology Unit, Fondazione Policlinico Gemelli and Catholic University | Rome, Italy | Child Neurologist & Psychiatrist |
| Roberta Shepherd | The University of Sydney | Sydney, NSW, Australia | Physical Therapist |
| Alicia Spittle | University of Melbourne, and Murdoch Childrens Research Institute | Melbourne, Vic, Australia | Physical Therapist |
| Marelle Thornton | Cerebral Palsy Alliance | Sydney, NSW, Australia | Teacher |
| Jane Valentine | Princess Margaret Hospital, University of Western Australia | Perth, WA, Australia | Rehabilitation Specialist |
| Karen Walker | Cerebral Palsy Alliance, The University of Sydney, Children’s Hospital Westmead, The University of Sydney | Sydney, NSW, Australia | Nurse |
| Rob White | Cerebral Palsy Alliance | Sydney, NSW, Australia | Psychologist |

Feedback/Comments
The American Academy for Cerebral Palsy and Developmental Medicine has developed care pathways to assist the busy clinician. Please submit any advice or constructive feedback to make this pathway more useful.
NOTE: Feedback will be directed to the AACPDM Care Pathway Taskforce to review and consider on a queue 6-month basis.


